Denver, CO, May 02, 2017 –(PR.com)– cliexa-RA, cliexa™’s rheumatoid arthritis app, has been scored among the 19 best RA apps selected from 700+ apps to NIH research:
//www.ncbi.nlm.nih.gov/pmc/articles/PMC5340922/
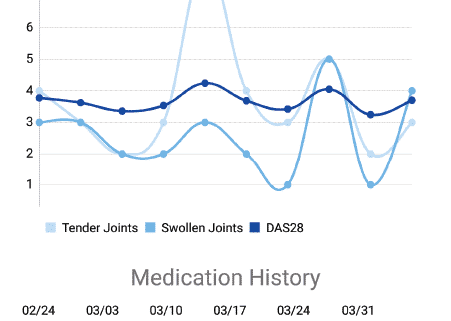
The review highlights which apps weigh ACR/EULAR treatment guidelines and which include DAS28, among other important features. Researchers rank the apps using the Mobile Application Rating Scale (MARS). A scientific review of mobile apps for tracking rheumatoid arthritis disease activity finds that most apps are either simple calculators for physicians to measure disease activity or tools for patients to track symptoms, most of which do not uniformly collect data using validated instruments or composite disease activity measures.
cliexa’s Co-Founder and CEO Mehmet Kazgan says “cliexa™ changes doctor and patient behavior by connecting patients, clinicians and insurers to better care for and manage chronic conditions which translates into significant cost savings, a better doctor and patient experiences and better outcomes yielding higher customer defined value.” RA Medical Director of cliexa, Dr. Spencer stated that, “In today’s demanding medical environment physicians have limited time to gather relevant data, much less calculate an activity index. With an easily used technology application, patients can be instrumental in helping their doctors determine their disease activity, which, in turn, can lead to better informed treatment decisions. With cliexa-RA we are offering a free mobile application to achieve this goal.”
About cliexa
cliexa staff consists of executive healthcare professionals, physicians, scientists and technologists. cliexa’s first mobile platform, cliexa-RA for Rheumatoid Arthritis has been released to Apple Store in January 2016 and since then, cliexa has developed 3 disease tracking applications, both in iOS and Android platform for Rheumatoid Arthritis (cliexa-RA), Inflammatory Bowel Diseases (cliexa-IBD) and Chronic Obstructive Pulmonary Disease (cliexa-OPD). In November 2016, cliexa has partnered with nation’s biggest payor and provider to run a clinical study with cliexa-RA and raised more than $500K. cliexa platform is a SaaS model offered to end users, clinics, payors and pharma companies for tracking chronic disease symptoms and analytics.
Newswire Official Press Release
cliexa hosted a booth at Rocky Mountain Gastroenterology’s 15th Annual Pow Wow Conference on Saturday, April 9, 2016. Conference was held at Hyatt Regency at Denver Tech Center, Denver, CO.
The GI Pow Wow is designed to provide a gastroenterology & hepatology update to community primary care providers, surgeons, specialists, as well as medical oncologists & radiologists from throughout Colorado and the surrounding region.
Topics included:
- Opioid receptors & implications on treating Intestinal Disorders
- Childhood Obesity & Fatty Liver Treatment
- IBD Treatment update
- HCV update
- Esophageal Cancer Prevention
- Drug-Induced Liver Disease
At a conference with 400 healthcare industry providers, cliexa had a lot of visitors with lot of questions for cliexa-RA which is in Apple store and cliexa-IBD in early May 2016. Lots of physicians showed interest at scientific disease activity scoring which is tracked with both cliexa-RA and cliexa-IBD dynamically.
Our CTO Nathan Blair, CSO Esra Nutku-Bilir and myself have answered questions from different providers. cliexa-IBD for Inflammatory Bowel Disease is using a survey type disease activity scoring whereas cliexa-RA uses DAS28 joint point calculation.
cliexa-IBD has attracted lots of physicians, who are interested in EMR integration with their current systems which is now part of cliexa’s roadmap.
Here are some photos from the exhibition!
Mehmet Kazgan, CEO.