Rheumatoid arthritis (RA) is a chronic inflammatory disease that may impair daily functioning and quality of life due to pain, swelling and stiffness. The disease has an unpredictable course and the main treatment goal is to suppress disease activity in order to prevent joint damage and to improve daily living. Treatment of RA mainly includes disease modifying anti-rheumatoid drugs including biologicals. Both international and national treatment guidelines recommended assessing the disease activity using measures such as DAS28, and doing regular follow up assessments, in which the level of activity dictates the frequency of monitoring. Frequent and regular assessments, however, may not be possible in a clinical practice because of a busy daily schedule.
Healthcare data suggest that Patient Reported Outcomes (PROs) have certain significance for routine clinical care. Following are the questions that we will be discussing here: What is the validity of patient self-report outcomes to guide a treat-to-target strategy in clinical trials and usual clinical care of rheumatoid arthritis? Which measures to use? How could physicians (Rheumatologists) use these measures in a daily clinical practice? Challenges involved, and why DAS28? How could patients contribute to their own treatment?
What is the validity of patient self-report outcomes in clinical practice?
It seems attractive to assess disease activity from patient’s perspective using validated patient reported outcomes (PROs) as an alternative or as an addition to laborious frequent joint assessments. There is evidence concerning PROs in clinical care in documenting low disease activity and remission, including a meta-analysis of studies that document the value of using PROs to implement ‘treat-to-target.’ PROs are well established by groups of experts from the Outcome Measures in Rheumatoid Arthritis Clinical Trials –working group (Felson et al , 1993). Patient global health (pGH) a PRO element is included as a recommendation in the 2010 (ACR-EULAR guideline that proposed tighter definitions for clinical remission in clinical trials, and is now also established for clinical practice (Smolen et al, 2013).
Which measures to use?
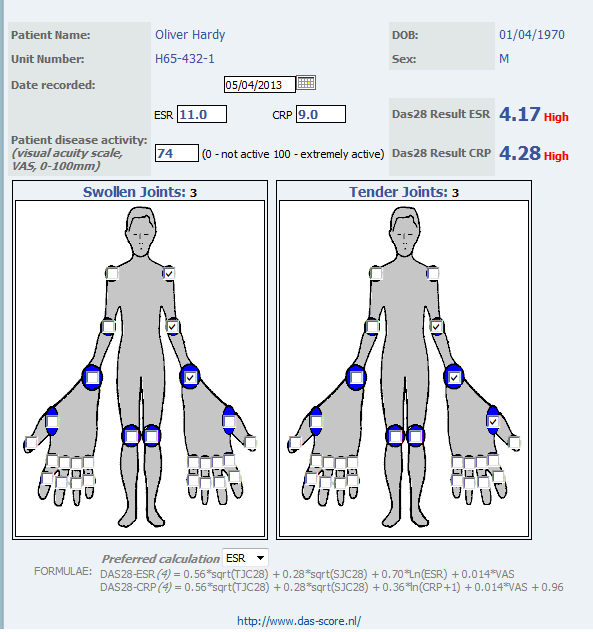
Of the 63 currently available RA disease activity measurement tools, the data may be filtered down to 6 recommended measures: the CDAI (clinical disease activity index), DAS28 (ESR and CRP; disease activity score with 28 joint counts) PAS (patient activity scales), PAS-II, RAPID-3 (routine assessment of patient index data), and SDAI (simplified disease activity index). All 6 measures produce a single consistent index and have defined ranges for indicating low, moderate, or high disease activity or clinical remission. Studies demonstrated that by applying these tools systemically in clinical practice, physicians would be able to treat to target and effectively implement the American College of Rheumatology recommendations for the treatment of RA (J. Anderson et al, 2012).
How could physicians (Rheumatologists) use these measures in a daily clinical practice? Challenges involved, and why DAS28?
Realizing the heterogeneity of settings in which health care is delivered to patients with RA in the US, there are selected measures that offer a full range of data collection options. Some are purely patient-reported (PAS, PAS-II, and RAPID3), some need physician assessment (CDAI), and some need physician and laboratory acute phase reactants (SDAI and DAS28). While most of these measures are considered as possible options, purely patient-reported measures may lack formal assessments. Therefore, patient-reported measures must be complemented by a careful joint examination, and do not prevent performance of a formal joint count or any other measure by a treating physician. Meanwhile, DAS28 calculations provide use of a continuous measure with absolute laboratory values and formal physician-assessment in daily clinical practice and clinical trials. Therefore DAS28 has been shown to a useful instrument to monitor disease activity in to titrate treatment with biologicals (Fransen et al, 2005). However, frequent collection of patient data and calculations are challenging in a busy rheumatology practice. There are indications that the DAS28 is not measured frequently enough in standard rheumatology care. Thus, daily, regular, patient self-report joint assessments may provide a useful, cost-effective method to complement physician’s role and implement treat-to-target in patients with RA.
How can you -as a patient- contribute to your own RA- treatment?
Collaborative Network 4 Clinical Excellence (CN4CE) has recently launched an application, cliexa-RA that is based on DAS28 score calculations with erythrocyte sedimentation rate (ESR). Cliexa-RA is available for iOS devices in the Apple store. This, so far unique, application is designed to combine patient-reported measures, with laboratory (ESR values) and provides an essential tool for the physician to review quickly and monitor disease activity, and make a medication titration, even within a busy schedule. This application is designed to collect patient self-reported joint symptom data and medications, daily and frequently, and calculates DAS28 scores automatically by combining the other variables involved in DAS28 scoring system (such as ESR). Physicians act to complement patient’s role by reviewing and confirming the data. Thus, cliexa-RA provides disease activity monitoring tool based on DAS28 scoring system and data on the medication history over a time period. cliexa-RA not only increase awareness, and encourages patients to involve in their own treatment, but also provides an effective support tool for the physicians’ office to increase treatment outcomes and implement treat-to-target in patients with RA.
Esra Nutku-Bilir MD, PhD
Co-Founder, Scientific Advisor
Post Doctoral Award recipient from American Rheumatoid Arthritis Foundation