Digital health refers to the use of information and communications technologies to exchange medical information. The intended uses vary, but typically focus on improving outcomes, convenience and workflow, lowering costs and improving the doctor and patient experience, engagement or loyalty.
There are many different kinds of digital health technologies used to accomplish different intended uses, and , consequently, the rates of adoption and penetration by doctors therefore varies depending on which technology is being measured, for example, for example real time telemedicine v store and forward telemedicine v remote sensing.
Here’s how I slice and dice the industry:
1. Remote sensing and wearables
2. Telemedicine
3. Data analytics and intelligence, predictive modeling
4. Health and wellness behavior modification tools
5. Bioinformatics tools (-omics)
6. Medical social media
7. Digitized health record platforms
8. Patient -physician patient portals and consumer experience
9. DIY diagnostics, compliance and treatments
10. Decision support systems
11. Population health
12. Workflow improvement
Here are some basics about dissemination and implementation science.
Here are the ABCDEs of technology adoption.
The present status of doctors using these products and services looks something like this:
- 40% of physicians believe that utilizing digital technologies to keep track of and communicate with patients will lead to better health outcomes.
- 47% of physicians who owned a smartphone used the device to show patients images and videos.
- More than 33% of doctors recommended their patients to utilize mobile health applications.
- More than 20% of doctors have integrated and utilized secure messaging platforms to speak to their patients.
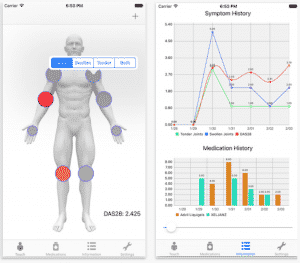
- More than 20% of physicians monitored patients remotely with an average oversight of 22 patients per month.
- Nearly half of 250 surveyed doctors are not prescribing mHealth apps to their patients due to the few regulations and mobile security policies in place within the mobile health sector.
Overall, 33% of physicians surveyed said they were using some form of telemedicine and another 29 percent said they were planning to, making a total of 62 either using or considering telemedicine, defined as “care via telephone, video visits, web cam visits – or other consultations not in person”.
However, when practitioners were asked “[d]o you have a mechanism to get paid for telemedicine services — are you in a network that will reimburse for that?”, only 19 percent said yes.
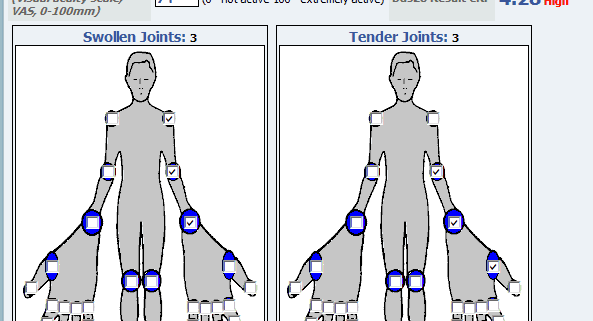
Just 18% of hospitals participating in a survey always use PROs (patient reported outcomes) as part of the care process, although an additional 72 percent said they are working on integrating patient-provided data into their routines within the next three years.
Among the organizations that are using PROs to some degree, 59% engage in chronic disease management and 58% utilize the data for surgical interventions and post-surgical patient tracking. Just over a quarter of providers focus on the use of PROs for mental healthcare, while 22% use the data for treating and managing cancer patients.
Here are some health technology gaps.
Here’s why telemedicine has not reached the tipping point.
Measuring digital health adoption and penetration will vary, of course, on who you sample and how. For example, the digital health practice patterns of a two person independent, rural general practice will probably vary substantially from that of an academic surgical or medical specialty group practicing tertiary referral medicine with backing of a large integrated delivery network budget. BIG MEDICINE, and arguably, their digital health needs, is different than small medicine.
For digital health to cross the chasm and reach wider adoption and penetration, the rules need to change, trust levels need to rise and entrepreneurs need to get smarter about when to use a given technology for an intended use where there is a clear pain point that begs for a solution.
Arlen Meyers, MD, MBA is the President and CEO of the Society of Physician Entrepreneurs, and the CMO of cliexa.